
Stratification factors included PD-L1 expression (≥1% versus <1%), choice of taxane (paclitaxel versus nab-paclitaxel) and geographic region (East Asia versus rest of the world). In case of no disease progression after four cycles of the combination strategy, maintenance treatment either with pembrolizumab or placebo was administered for up to 35 cycles.
#KEYNOTE 407 TRIAL#
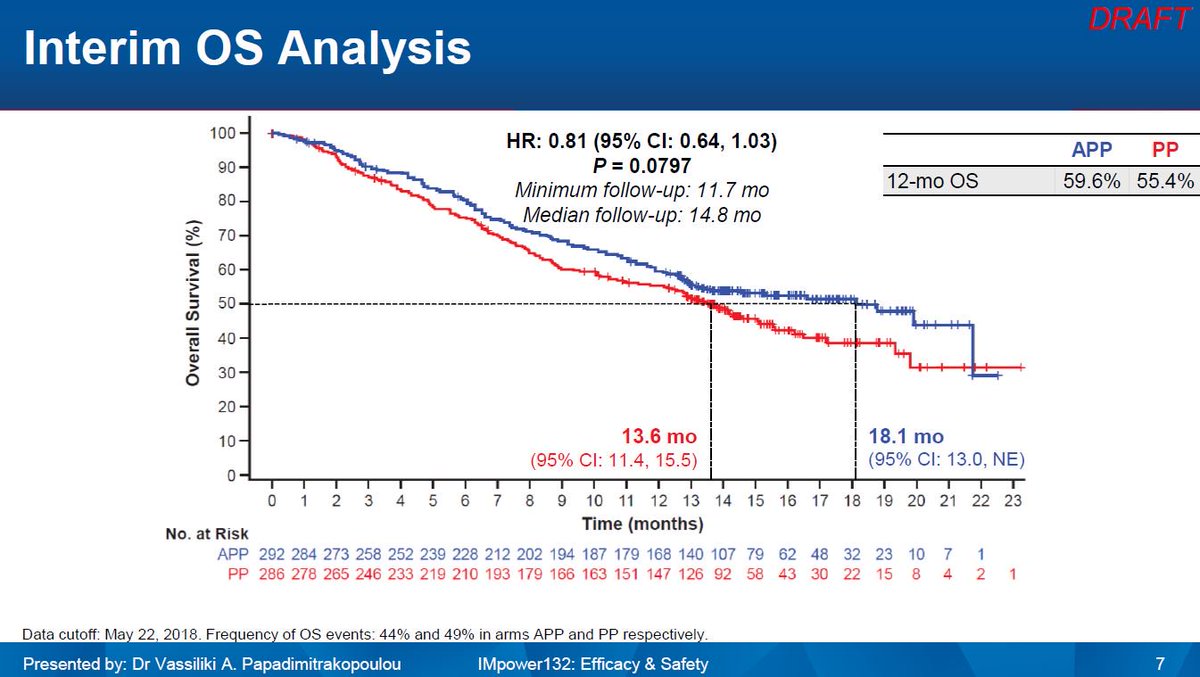
The phase III, placebo-controlled, KEYNOTE-407 trial assessed the progression free survival (PFS), assessed by independent radiological review, and overall survival (OS) benefit of adding pembrolizumab to carboplatin plus (nab)-paclitaxel in 559 treatment-naive metastatic squamous NSCLC patients. However, based on the KEYNOTE-407 trial, the introduction of ICI in the first-line treatment of metastatic squamous NSCLC has marked a step forward in the therapeutic strategy of this patient population, improving both the outcome ( 1, 2, 10) and quality of life ( 11, 12). In contrast to non-squamous histology where personalised treatment approaches along with ICI have improved the outcome ( 2, 4, 8), survival improvements in squamous NSCLC have been quite limited or without any meaningful clinical impact so far ( 9). In the first-line setting, ICI have become standard of care (SoC) either as monotherapy (pembrolizumab for tumours with high programmed cell death ligand-1 (PD-L1) expression (≥50%) in Europe or with PD-L1 ≥1% in the United States of America) ( 1- 3) regardless of histologic subtype, or in combination with chemotherapy regardless of PD-L1 expression in non-squamous (pembrolizumab/atezolizumab plus platinum-doublet chemotherapy, or atezolizumab-carboplatin-paclitaxel-bevacizumab) ( 4- 7). Immune checkpoint inhibitors (ICI) have improved the prognosis and redefined the treatment strategy of metastatic non-small cell lung cancer (NSCLC) patients.


 0 kommentar(er)
0 kommentar(er)
